The process of electrolytic plasma polishing
The process of electrolytic plasma polishing has been industrially usable fairly recently and is still relatively unknown. The following should explain the process of plasma polishing and the resulting benefits of it. For a more detailed description please follow the links at the end of each section.




Procedure
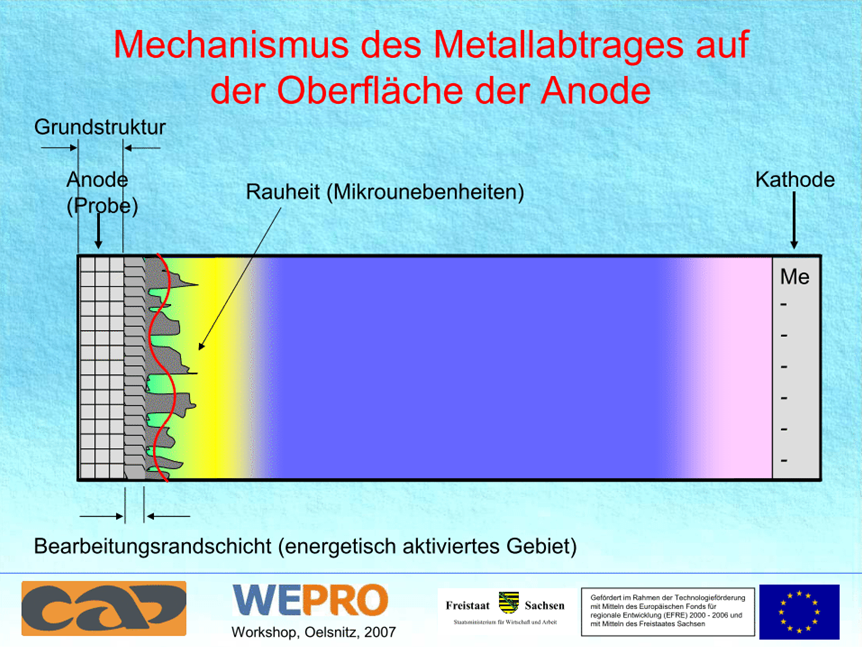
Anodically polarized metallic workpieces are placed in an electrolytic bath for the plasma polishing process. The electrolyte consists of a solution of about 98% water and 2-3% salt. This produces gas which wetted the workpiece to be polished and results in process-induced development of plasma. This plasma coats the workpiece and leads to a reduction of the roughness as well as removes organic and inorganic contaminants with minimal mass loss. Depending on the material specifications the typical material removal lies between 2 - 8 µm per minute. The geometric shape of the component is almost maintained.
Electrolytes are used to date for treatment of:
| Groups | Materials |
|---|---|
| Ferrous metals: | all stainless steels (polishing depends on the degree of alloying elements and carbon content) |
| Non-ferrous metals: | brass and its alloys Chromium-cobalt alloys Chromium-cobalt-molybdenum alloys Tungsten Molybdenum |
| Light metals: | Titanium and Titanium alloys some Magnesium alloys |
For a more detailed description of the electrolytic plasma polishing process and a comparison with conventional electropolishing processes.
The innovative process of plasma polishing lends itself as the solution in areas such as surface polish, deburring and purification due to its many benefits like:
- levelling of microroughness (< 0,01 µm)
- minimal material removal
- processing of any contours is possible
- achievement of unprecedented degree of gloss
- no prior treatment or cleaning of the workpieces necessary
- the environmentally friendly electrolytes consists 98% H2O
- no use of environmentally harmful substances or highly concentrated acids
- once processed surfaces are more corrosion resistant than its initial state
- lowest thermal and mechanical stress on the surface ( t < 100°C )
- no cytotoxic effects can be expected on plasma polished surfaces
For a more detailed description of the benefits of the electrolytic plasma polishing process please [Link]
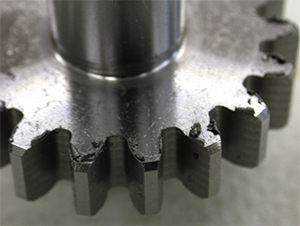
Prior to treatment

Outcome after plasma polishing
Plasma Polishing process description and the significant differences to electropolishing
Glossy surfaces for aesthetic and functional reasons are becoming increasingly important. A number of residues must be removed to generate high-gloss surfaces, which form during the manufacturing process of these parts. They form for example due to residues from the casting process, machining marks or from layers resulting from heat treatments or welding processes.
For those reasons there are polishing operations required, which provide surfaces without grooves and other structural defects in the surface geometry. Currently polishing effects are largely achieved by mechanical or chemical procedures and lately also by laser assisted processes. Each of these processes only applies to a limited set of materials and has restrictions with regards to costs, processing time and environmental impact.
A new method called Plasma Polishing is suitable to overcome the disadvantages of the conventional polishing methods.
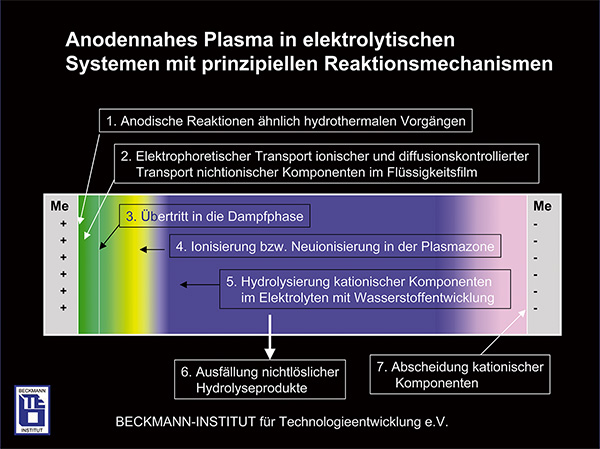
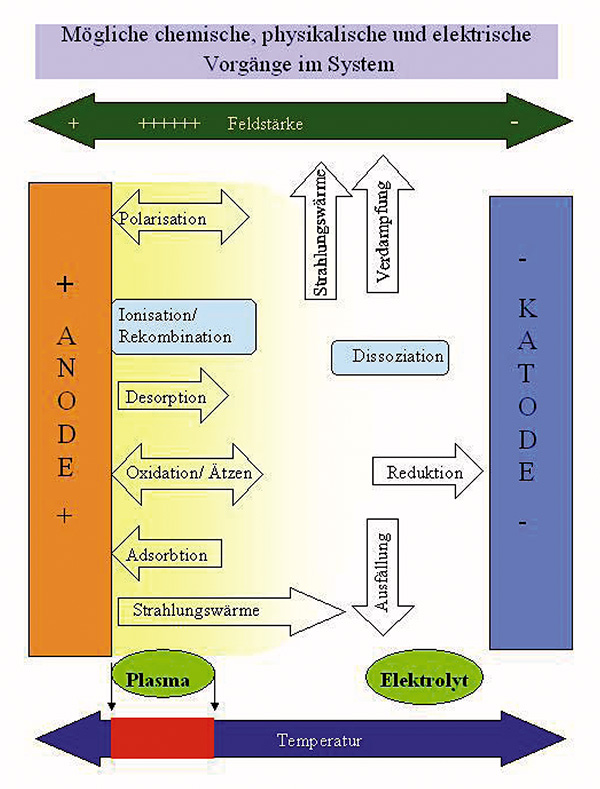
Plasma Polishing represents a new approach for polishing and is based of a physical- chemical effect, which happens on the surface of electrodes of an electrolytic system in conjunction with high voltage. Plasma Polishing is an electrolytic process by which anodically polarized metal components through the process-induced formation of a plasma membrane caused by thermal and electrochemical processes achieve an unprecedented gloss that could not be reached by conventional polishing processes.
Local gas development of the electrolyte at the smaller electrode (the part serving as an anode) leads to a gas stream, which coats the entire surface of the workpiece and enables the formation of the plasma membrane. In addition to the levelling of microroughness with minimal mass loss, it also removes burrs as well as organic and inorganic surface contaminants. Furthermore, you can observe a respectable corrosion inhibition depending on the material on plasma polished surfaces. Plasma polished metal can be characterized by its improved corrosion protection compared to the initial state.
This process is a supplement or problem solver of existing surface treatments with its attainable roughness values of less than 0,01 µm. The typical material remove during plasma polishing is 4-8 µm / minute depending the material specification and therefore smaller than electropolishing. The metal parts in the plasma polishing bath will not increase their temperature beyond 100 °C, also not superficially.
The process is well suited for polishing and deburring of milling, turning and investment castings. This process ensures dimensional stability and minimal chamfering to maintain the required tolerances. The surfaces of hardened components are polished. The plasma thermally brought in motion causes a combustion of organic surface layers by the oxidizing character of the process. But also inorganic substances on the surface can be oxidized and removed if the evaporating temperature or decomposition temperature of the products of reaction is less than 2000K.
This process is a supplement or problem solver of existing surface treatments processes with its attainable roughness values of less than 0,01 µm.
Suitable electrolytes have been developed for the following materials:
| Groups | Matirials |
|---|---|
| Ferrous metals: | all stainless steels, polish degree depends on the degree of alloying elements and carbon content |
| Non-ferrous metals: | Chromium-cobalt alloys Chromium-cobalt-molybdenum alloys Tungsten Molybdenum |
| Light metals: | Titanium and Titanium alloys some Magnesium alloys |
Some metal composites and MIM produced process parts.
About the Plasma Polishing Process
The plasma polishing process is similar to electropolishing in its machine construction. The part that is to be polished is electrically contacted to be used as an anode and immersed in an electrolytic bath in both cases.
There are three main differences to the electropolishing processThe firstly is the electric bath voltage which needs to be high above 200 volts to ignite the plasma under water. The resulting surface current density is comparable to electro polishing. However the typical material removal with plasma polishing of 1 µm / minute is 10 to 30 times smaller than electro polishing.
The Second major difference is the electrolyte composition, which requires only a small proportion of dissolved salts in water for the plasma polishing process.
And finally the third difference is that we have so far been able to find non-toxic salt combinations to create stable plasma to produce the special polishing effect. Since plasma polishing only uses environmentally friendly chemicals it will avoid any problems with hazardous working conditions compared to traditional polishing methods. Moreover, plasma polishing is environmentally friendly by using non-toxic chemicals in low concentration. Any development of additional material specific electrolytes will follow the concept of environmental impact, possible material contamination and disposal.
Special features:
Metal parts never heat up beyond 100 °C not even superficially during the process of plasma polishing.
Plasma polished metal is characterized by an improved corrosion protection compared to the initial state.
Chemical used:
- Ammonium sulfate with water-soluble sulfur < 10 %
- optional: Phosphoric acid approx. 10% (only for removing stains)
- deionized water
Polishing current is dependant on the working surface current density: 0.22 A/cm²
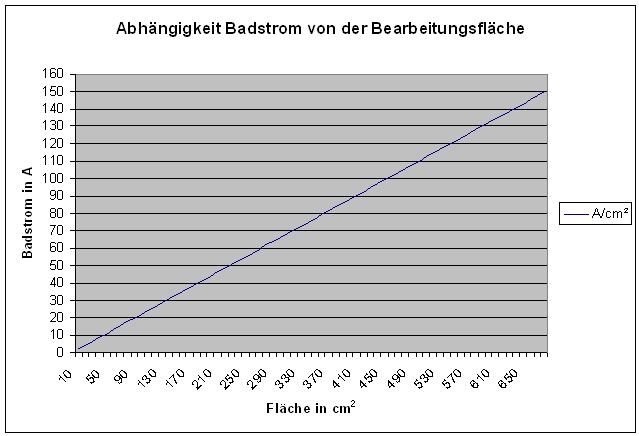
The polishing current is directly related to the to be polished surface of the component.
Summary of the processed stainless steels
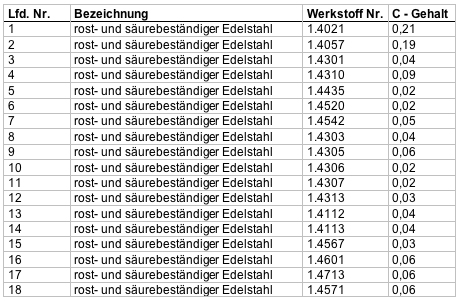
The range of stainless steels that can be processed includes all material specifications, the material removal and the degree of surface gloss which is dependent of the respected alloy components.
Process description of Electro Polishing Fundamentals of Electro Polishing
Electro polishing is defined as the electrochemical treatment which leads to levelling, shine and passivation of a metal surface that was originally dull and rough. Electro polishing is associated with the electrically abrasive manufacturing methods according to DIN 8580. It is the electrochemical removal of a surface as a result of the electric charge transfer between a metal workpiece and a liquid medium, the electrolyte.
In principle, electro polishing is a reverse galvanic process under which the workpiece is connected to the positive pol of a DC voltage source (Anode). Parts of the materials surface dissolve under the influence of the direct current of the electrolyte. The surface is smoothed, leveled and passivated.
Significant benefits or special features of electro polishing of stainless steel workpieces are the achievements of optical and technical surface properties. The essential characteristic of electro polishing is the abrasion of asperities which at first leads to a decrease in micro-roughness and with prolonged treatment time it can reduce the micro-roughness. The preferred abrasion of material acutes causes workpiece edges to be removed faster than the rest of the surface and the consequences of the mechanical processing causes fine burrs to be removed. This is known as deburring by electro polishing. Also the corrosion resistance of stainless steel is favorably influenced by eletrco polishing. Since corrosion depends on the surface of a material and thus the size of the surface, especially on a micro level, it has a particular importance. In addition, electro polishing of stainless steel is connected to a passivation effect, because the anodic circuit offers oxygen very strongly to the workpiece. Chromium oxide can therefore develop full and thick on the surface which is inevitable for the corrosion resistance. Electro polished stainless steel surfaces offer great benefits from a hygienic standpoint as it also removes germs and pathogens during the cleaning and passivation and the development of micro-organisms is greatly reduced. This is especially important for use in public areas auch as hospitals and in the food industry.
Workpieces should meet the following requirements for electro polishing:
- advantageous workpiece geometry - bulk capabilities for mass parts
- walls should not be too thin - contact points for power supply
- highly electrically conductive surface
- mechanically pre-processed surface
Additional openings or ventilation holes need to be inserted so that cavities can be filled completely with the electrolyte and subsequent removal of gases produced during electro polishing and avoiding gas pockets can be achieved. Narrow gaps, seams and beadings are difficult to clean from invading acids and therefore should be avoided. It should also be considered with regards to adherence tolerance that the typical material removal during the electro polishing is usually 10 to 30 µm which might need to be limited by appropriate means.
Essential for good electro polishing is a thorough pre-cleaning of the workpieces. Particularly damaging are lubricants and oxide layers for electro polishing. Light oxide films such as tarnish from the glowing stainless steel can be removed easily. Scale residues must always be removed before electro polishing.
Electro polishing of stainless steel is mainly achieved in electrolytes from highly concentrated mixtures of phosphoric acid and sulfuric acid with additives to improve gloss, smooth leveling and efficiency. The electrolytes do not engage with the stainless steel in the electro less state. Most electrolytes for electro polishing have been developed in the United States and France and some of them are patented. The water content is used for optimizing the conductivity and the polishing effect. Electro polishing plants are usually baths with heating, cooling and electricity corresponding fittings.
Power is supplied by an infinitely variable rectifier. The bath is usually about 10 m in length depending on the applications. The parts need to be rinsed with running water and cleaned to remove all the electrolytes after the electro polishing. This can be done in several steps to completely remove all acid residues.
Technology
Highly polished surfaces for functional and aesthetic applications are becoming increasingly important in many areas. A number of residues must be removed that were generated during the manufacturing process of these parts to achieve the high-gloss surfaces. Residue can occur for example during the casting process, by machining marks or layers resulting from heat treatment or welding processes.
This is the reason why polishing operations are required, which provide surfaces without grooves and other structural defects to the surface geometry. Currently polishing effects can be achieved by mechanical, chemical or electrochemical processes as well as more recently by laser-assisted methods. Each of these methods can only be used for a limited choice of materials and has restrictions regarding cost, processing time and environmental impact. A new innovative technology to meet the high requirements in the field of surface treatments was developed by the plasotec GmbH with the electrolytic plasma polishing process.

Benefits
The innovative method of plasma polishing offers a number of benefits that allow it to be used for different application areas. The unique features of plasma polishing make this technology particularly suited as a problem solver for polishing, cleaning and deburring of metallic surfaces.
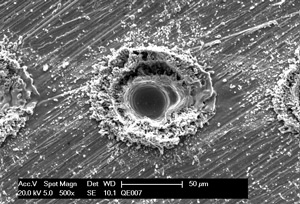
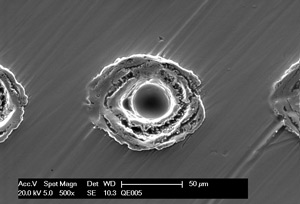
- levelling of micro roughness (< 0,01 µm)
- typical material removal 2 - 8 µm / minute (10 to 30 times smaller than electro polishing)
- processing of any contours is possible
- achievement of unprecedented degree of gloss (especially for stainless steels)
- no prior treatment or special cleaning of the workpieces necessary
- environmentally friendly electrolytes consists of 98% H2O, no use of environmentally harmful substances or highly concentrated acids
- once processed surfaces are more corrosion resistant than its initial state (depending on material specifications)
- lowest thermal and mechanical stress on the surface ( t < 100°C )
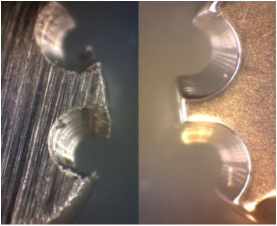
No cytotoxic effects can be expected on plasma polished surfaces.